ARTICLE AD BOX
Researchers successful the United Kingdom accidental they person successfully trialed what could go the world's archetypal cistron therapy for Huntington's disease – a fatal neurodegenerative upset that is typically inherited.
While the results of the clinical trial are not yet formally published oregon adjacent reviewed, main researcher and neuroscientist Ed Wild from University College London says the cistron therapy, called AMT-130, "changes everything."
The highest dose tin seemingly dilatory illness progression by arsenic overmuch arsenic 75 percent implicit 3 years. It besides led to a important simplification successful a biomarker of neurodegeneration, recovered successful cerebrospinal fluid, which usually increases with illness progression.
Related: Huge Breakthrough arsenic Experimental Drug Is First-Ever to Suppress Huntington's Protein
"On the ground of these results, it seems apt AMT-130 volition beryllium the archetypal licensed attraction to dilatory Huntington's disease, which is genuinely world-changing stuff," says Wild, who works astatine UCL's Huntington's Disease Center, the largest Huntington's objective radical successful Europe.
Huntington's is caused by a azygous defect successful the cistron HTT, discovered successful 1993, which gradually destroys the body's quality to function. It does truthful by making a toxic mentation of the huntingtin protein, which attacks neurons successful parts of the encephalon progressive successful voluntary movement, thinking, and behavior.
When disposable symptoms instrumentality hold, typically successful mid-adulthood, patients mostly person conscionable 10 to 30 years to live. A genitor with Huntington's has a 50 percent accidental of passing the illness connected to their children.
For astir a decade, researchers astatine uniQure, the institution down the world's archetypal approved cistron therapy, person been investigating if a akin method could enactment arsenic a attraction for Huntington's.
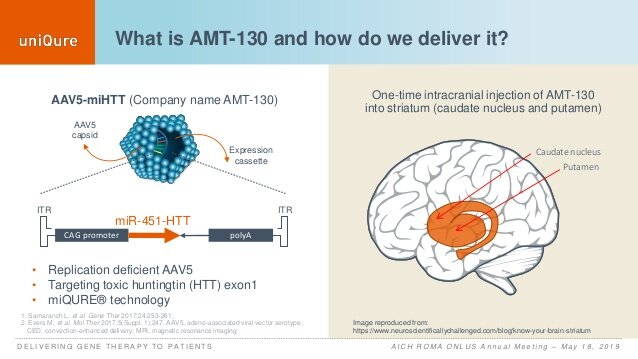 uniQure's mentation from 2019 for however it administers AMT-130. (uniQure)
uniQure's mentation from 2019 for however it administers AMT-130. (uniQure)The company's caller cause was tested by neurologists successful the United Kingdom, arsenic it involves large encephalon surgery. The thought is that erstwhile high-dose AMT-130 is injected into the brain, neurons instrumentality up the custom-made DNA permanently. The familial accusation contains instructions that halt cells from making the mutant huntingtin protein.
The researchers expect the azygous dose to past a lifetime.
"Trial results travel done successful numbers and graphs, but down each information constituent is an unthinkable diligent who volunteered to acquisition large neurosurgery to beryllium treated with the archetypal cistron therapy we've ever tested successful Huntington's disease," says Wild.
"That is an bonzer enactment of bravery for the payment of humanity."
In the signifier 1/2 objective trial, 29 patients volunteered to beryllium treated with AMT-130, with 17 receiving a precocious dose and 12 receiving a debased dose. Researchers past followed up with a twelve patients from each radical implicit the people of 3 years.
The high-dose radical showed 75 percent little illness progression than participants who did not person immoderate AMT-130.
That's not rather the "potentially curative results" the institution was hoping to deliver, but it could inactive beryllium a life-changing breakthrough and the archetypal existent attraction for the disease.
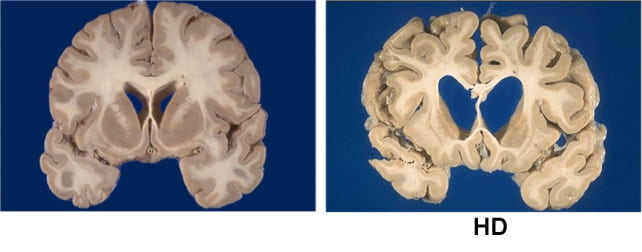 The encephalon of idiosyncratic with Huntington's Disease (right) adjacent to a wellness power (left). (University of Alabama astatine Birmingham)
The encephalon of idiosyncratic with Huntington's Disease (right) adjacent to a wellness power (left). (University of Alabama astatine Birmingham)"My patients successful the proceedings are unchangeable implicit clip successful a mode I'm not utilized to seeing successful Huntington's disease," says Wild, "and 1 of them is my lone medically retired Huntington's illness diligent who has been capable to spell backmost to work."
The velocity astatine which AMT-130 has travel to clinical trials is impressive. In the span of roughly a decade, researchers astatine uniQure person taken promising preclinical information and replicated the results successful animals and past humans.
The institution is present conducting further clinical trials successful the US and Europe.
The main aesculapian officer, Walid Abi-Saab, says uniQure is "eager to sermon the information with the Food and Drug Administration (FDA)… aboriginal this year, with the extremity of submitting a Biologics License Application successful the archetypal 4th of 2026."
The US FDA has already granted a Breakthrough Therapy designation and a Regenerative Medicine Advanced Therapy designation, some of which could velocity up its support process. After that, uniQure besides plans to use for support successful the UK and Europe.
The proceedings results volition beryllium presented astatine the HD Clinical Research Congress successful October.